Quality Risk Management

Introduction
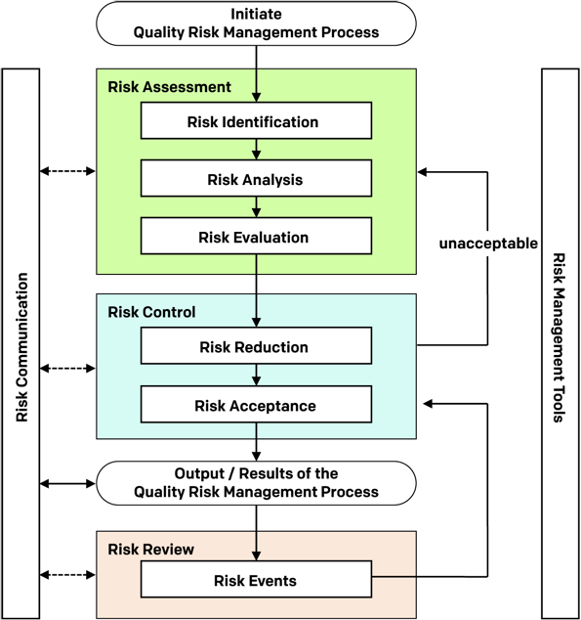
In 2005, the International Conference on Harmonisation’s (ICH’s) ‘’Q9’’, aimed at providing the biopharmaceutical sector with guidance towards a science and risk-based approach to quality management was published. The concepts outlined in ICH Q9 focused on the fact that evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient. ICH Q9, Quality Risk Management, represents the first internationally recognized guideline specifically addressing QRM for the pharmaceutical and biopharmaceutical industries.
The purpose of ICH Q9 is to offer a systematic approach to quality risk management (QRM). It serves as a foundation or resource document that is independent of yet supports other ICH Quality documents and complements existing quality practices, requirements, standards, and guidelines within the biopharmaceutical sector and regulatory environment.
It specifically provides guidance on the principles and some of the tools of quality risk management that can enable more effective and consistent risk-based decisions, by both regulators and industry, regarding the quality of drug substances and drug products across the product lifecycle. ICH Q9 is not intended to create any new expectations beyond the current regulatory requirements (FDA, n.d.).
PRST, which was established in direct response to the drive for a paradigm shift in quality from the international regulatory community, started a research model that requires proactive engagement with global industry and regulators in order to explore and address the challenges and opportunities of implementing science and risk-based manufacturing approaches. Several of PRST’s researchers exploring the concept and application of QRM in pharmaceutical and biopharmaceutical companies and its effectiveness at managing risk to the patient. Following is a high-level guide to the QRM research conducted by the PRST, followed by helpful references on these studies and additional relevant information.
The first of these researchers was Kevin O’Donnell, a GMP inspector with the HPRA (previously known as IMB).. In 2004, IMB recognized that there was no adequate risk assessment or risk management tool available to the industry, which adequately addressed the risk-based GMP requirements of the EC Guide to GMP, particularly those requirements of Validation, Qualification & Change Control Activities.
In 2005, O’Donnell embarked on a PhD Research project to develop such a Risk-Based Management tool, and his Thesis was published in 2007, entitled:
‘The development of an Effective & Efficient GMP Risk Management Tool for use in Validation, Qualification & Change Control Activities within Pharmaceutical Manufacturing in the EU’
O’Donnell in his thesis states that in the pharmaceutical spaces risk is often considered an opposite of things which deliver benefit, (as in the so-called risk-benefit ratio of a particular pharmaceutical product), risk is considered to be associated with loss, not benefit, and with danger and potential negative consequences.
Throughout O’Donnell’s research, he published several seminal papers on QRM, which gave him global recognition as a leading regulatory voice in encouraging a risk-based approach to Pharmaceutical Manufacturing.
Relevant Publications
Links coming soon!
- EU GMPs Chapter 3, Chapter 5, Chapter 8 and Annex 15
- Mexican | NOM-059-SSA1-2015
- Japanese | Collection of Examples Related to GMP
- CFDA | Regulations of QRM
- PMDA| Guidance on the Manufacture of Sterile Pharma Products in using Aseptic Processing
- TGA PIC/S | Guide to GMP for Medicinal Products Annexes1
- ANVISA | Resolution – RDC Nº 17, OF 16.04.102
- Health Canada | Good Manufacturing Practices (GMP) Guidelines for Active Pharmaceutical Ingredients (APIs) [GUI-0104]
- ICH Q9: Quality Risk Management. Jun 2005.
- ICH Q8(R2): Pharmaceutical Development. Aug 2009.
- ICH Q10: Pharmaceutical Quality System. Jun 2008.
- Food and Drug Administration (FDA). Pharmaceutical cGMPs for the 21st Century – A Risk-based Approach (Final Report) 2004.
- Richter, Lori and Haddad, Ghada. Role of Senior Leadership in Quality Risk Management. Journal of Validation Technology, December 2015.
- Vesper, James and O’Donnell, Kevin. Current Challenges in Implementing Quality Risk Management. Pharmaceutical Engineering. November/December 2016.
Following O’Donnell’s research where the focus was on Risk Tool development, in 2014 Kelly Waldron joined the PRST as a PhD student to examine the Effectiveness and Maturity of Quality Risk Management in the industry. At the time, Waldron was the manager of Global Quality Risk Management based at Genzyme’s biopharmaceutical campus in Cambridge, Massachusetts, and was a renowned industry expert in the topic of QRM and regular speaker at international conferences. Waldron’s research examined barriers to the effective use of QRM within the pharmaceutical industry and developed a maturity framework for use by the industry to enhance the protection to the patient through more effective risk management.
Waldron, in her thesis titled “Managing risk to the patient: Recoding Quality Risk Management for the Pharmaceutical and Biopharmaceutical Industries,” discussed how ICH Q9 offered standard principles to be applied but did not provide specific guidance on the application of these principles; that the pharmaceutical and biotechnology industries still struggle with the “how to” and, most importantly, the roles and responsibilities associated with QRM.
However, although Waldron’s research did not explore in detail how individuals can contribute to QRM effectiveness, it identified the need for role-based competency in QRM and acknowledged that the traditional training model currently being employed in the pharmaceutical sector is inadequate in building such competency, to enable a mature state in the management of risk to the patient.
Waldron added that not all QRM practitioners require the same level of training. Competencies should be based on the level of involvement in QRM. She stated that all employees should be aware of the QRM principles and practices. However, specific roles should have customized training based on their level of interaction with QRM. While certain roles may require minimal technical knowledge of QRM, those who interact more frequently and deeply with QRM will require a commensurate level of training to ensure they can fulfill their responsibilities within the program.
Although ICH Q9 outlined multiple benefits of an effective QRM program, it was silent on the need to develop strategic and tactical skills for those involved in QRM.
This gap in competency building development for QRM practitioners and decision makers became more evident with the introduction of ICH Q10, “Pharmaceutical Quality System,” which characterized QRM as a necessary enabler of an effective quality management system. Looking at the currently available data, one could argue that the adoption of ICH Q9 and 10 have not really created the benefits it promised; the reasons for this have yet to be conclusively established but can be attributed to lack of role based competency models.
Without the critical QRM knowledge required to fulfil their role, those involved in applying the principles of QRM will not understand its benefit and will not be able to sustain it once implemented.
While training is critical to QRM, it only teaches basic concepts in order to execute a task (Greene & Calnan, 2015). What is needed is the development of a framework to define and advance individual QRM maturity. Using competency models from other sectors, assessment and certification can be used to address the biopharmaceutical sector’s needs and skill gaps in QRM.
ICH Q9 highlights the need for training of both biopharmaceutical sector and regulatory personnel in quality risk management processes, in order to provide them with a sufficient understanding of decision-making processes and to build confidence in quality risk management outcomes.
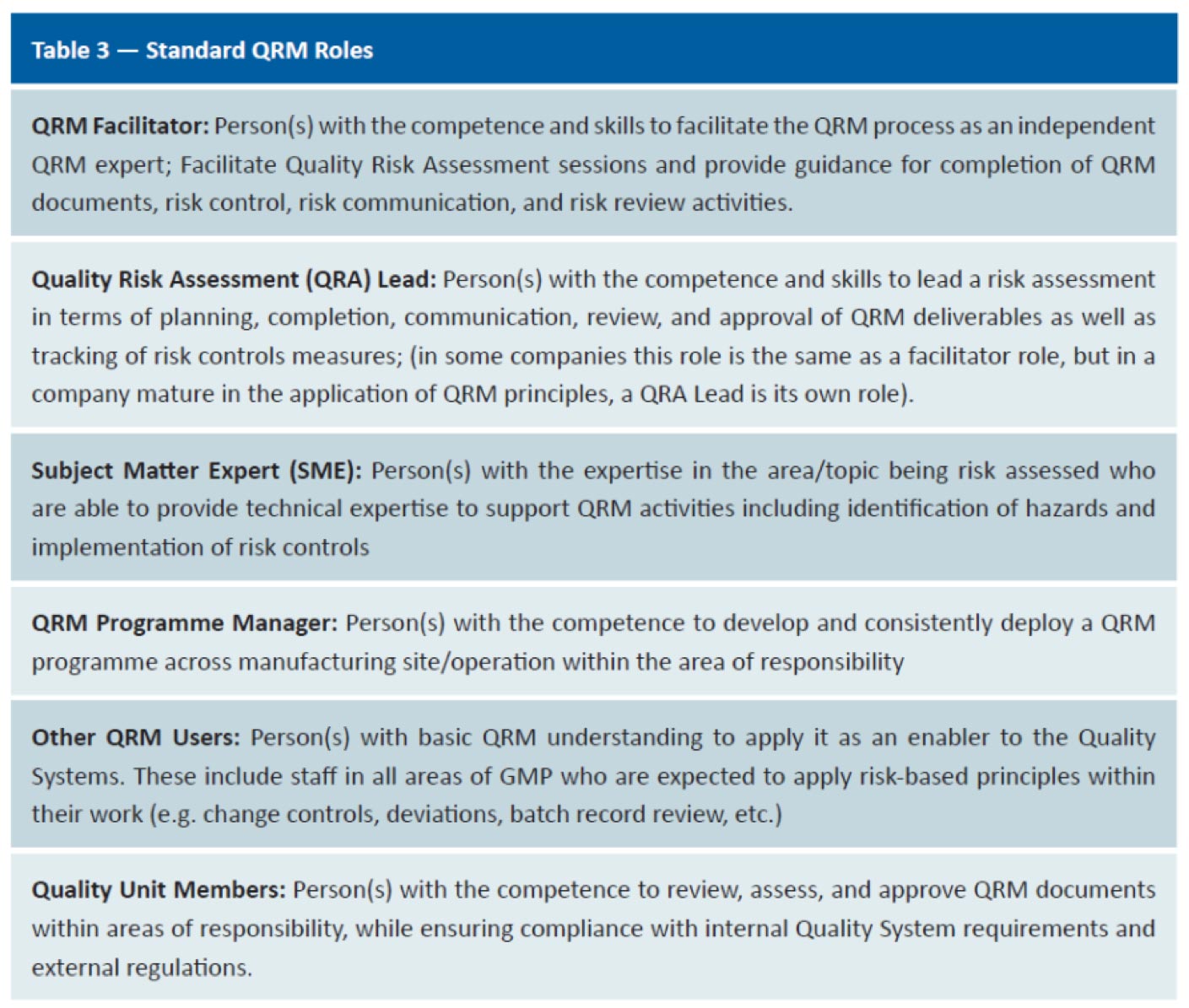
QRM role-based competency model for the biopharmaceutical sector. Using a modified Delphi research methodology, seven standard QRM roles were identified and were the basis for developing the QRM role-based competency model, which included technical and behavioural competencies associated with each of the seven QRM standard roles.
According to ICH Q10, QRM should be integrated into existing operations; this in effect means that QRM should be understood by all those involved in manufacturing operations and activities. Furthermore, ICH Q9 clearly indicates that both industry and regulators need to understand QRM, the tools available, and their formal and informal application, and that product and process understanding is required when applying QRM, and that statistical knowledge helps with understanding risk.
ICH Q9 touches on the role of personnel and how those involved in assessing deviations, investigations, and complaints should have QRM knowledge (e.g., change controllers and owners should understand QRM principles to evaluate impact of changes to product quality). In addition, regulators may use QRM principles in inspection activities to assist them with resource allocation, inspection planning and frequency, evaluating the significance of, quality defects, potential recalls and inspectional findings.
Byrne, a TU-Dublin graduate student, in her dissertation titled, “An Investigation into Quality Risk Management Knowledge Held By Junior Quality And Manufacturing Roles In The Pharmaceutical Industry” (May 2019) found that the pharmaceutical industry had not yet fully embraced QRM and has not yet fully understood the potential benefit of the analysis of risk to the patient. She stated however, that the pharmaceutical industry was seeking to increase the maturity of its QRM programmes and that an evaluation of current training procedures for QRM could prove beneficial in maturing a QRM programme. Byrne concluded from her research that incorporating QRM elements into GMP training should extend to all roles within an organisation to help progress the maturity of a programme and embed a quality risk management culture.
Following O’Donnell, Waldron, and Byrne’s work, Ghada Haddad, a pharmaceutical sector seasoned QRM subject matter expert, joined the PRST team with the goal of investigating the concept of QRM standard roles and associated role-based competencies within the biopharmaceutical manufacturing sector, to define standards and competencies to those involved in QRM.
Haddad in her thesis titled, identified 6 standard QRM roles, 7 QRM technical competencies and 4 behavioural competencies:
Technical
- Competencies for Risk Assessment
- Competencies for Rick Control
- Competencies for Risk Communication
- Competencies for Risk Review
- Competencies for Developing QRM Strategy, Programme Design, Policy and Procedures
- Competencies for Risk Culture
- Competencies for Developing Risk Performance and Reporting
Technical Competencies define what people have to know
and be able to do (knowledge and skills) to carry out their roles effectively. They are related to either generic roles (groups of similar roles), or to individual roles (role-specific co competences).
For example: a QRM Facilitator must have knowledge in the use of QRM Risk Assessment tools.
Behavioural
- Competencies based on Facilitation Skills
- Competencies based on Leadership Skills
- Competencies based on Decision Making Skills
- Competencies based on Communication Skills
These relate to types of behaviour which deliver effective results under such headings as team working, communication, leadership and decision making.
They are sometimes know as soft skills.
For example: if one needs to advocate risk management as a central part of an organisation’s strategic management then developing skills in influence and impact (a behavioural competency) would help achieve this.
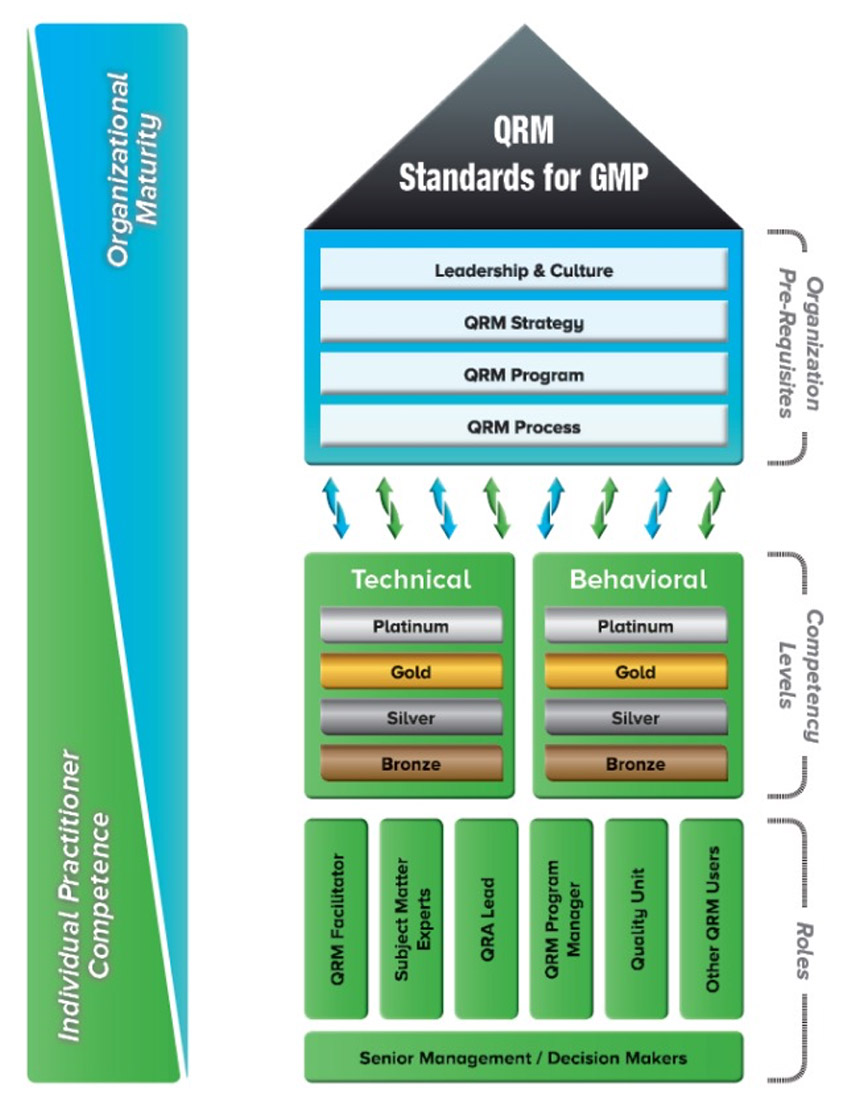
QRM Role-Based Competency Model
The QRM Role-Based Competency Model is divided into 3 sections:
- Organisation Pre-Requisites
- Competency Levels
- Roles
As the model is viewed from bottom to top, on the left hand of the model one can see how the competencies of the individuals blend into the organisational competencies.
Relevant Publications
- EU GMPs Chapter 3, Chapter 5, Chapter 8 and Annex 15
- Mexican | NOM-059-SSA1-2015
- Japanese | Collection of Examples Related to GMP
- CFDA | Regulations of QRM
- PMDA| Guidance on the Manufacture of Sterile Pharma Products in using Aseptic Processing
- TGA PIC/S | Guide to GMP for Medicinal Products Annexes1
- ANVISA | Resolution – RDC Nº 17, OF 16.04.102
- Health Canada | Good Manufacturing Practices (GMP) Guidelines for Active Pharmaceutical Ingredients (APIs) [GUI-0104]
- ICH Q9: Quality Risk Management. Jun 2005.
- ICH Q8(R2): Pharmaceutical Development. Aug 2009.
- ICH Q10: Pharmaceutical Quality System. Jun 2008.
- Food and Drug Administration (FDA). Pharmaceutical cGMPs for the 21st Century – A Risk-based Approach (Final Report) 2004.
- Richter, Lori and Haddad, Ghada. Role of Senior Leadership in Quality Risk Management. Journal of Validation Technology, December 2015.
- Vesper, James and O’Donnell, Kevin. Current Challenges in Implementing Quality Risk Management. Pharmaceutical Engineering. November/December 2016.