Pharmaceutical Quality System (PQS)
& Risk Based Decision Making

Introduction
In 2005, the pharmaceutical industry began its journey with Quality Risk Management (QRM) with the publication of a guidance by the International Council for Harmonisation (ICH). This body, formed to enhance convergence across global regulatory requirements, published ICH Q9: Quality Risk Management. The objective was to establish some principles and provide some tools to enable the application of QRM in a Pharmaceutical Quality System (PQS). The two primary principles of ICH Q9 are that:
- The evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient; and
- The level of effort, formality and documentation of the quality risk management process should be commensurate with the level of risk.
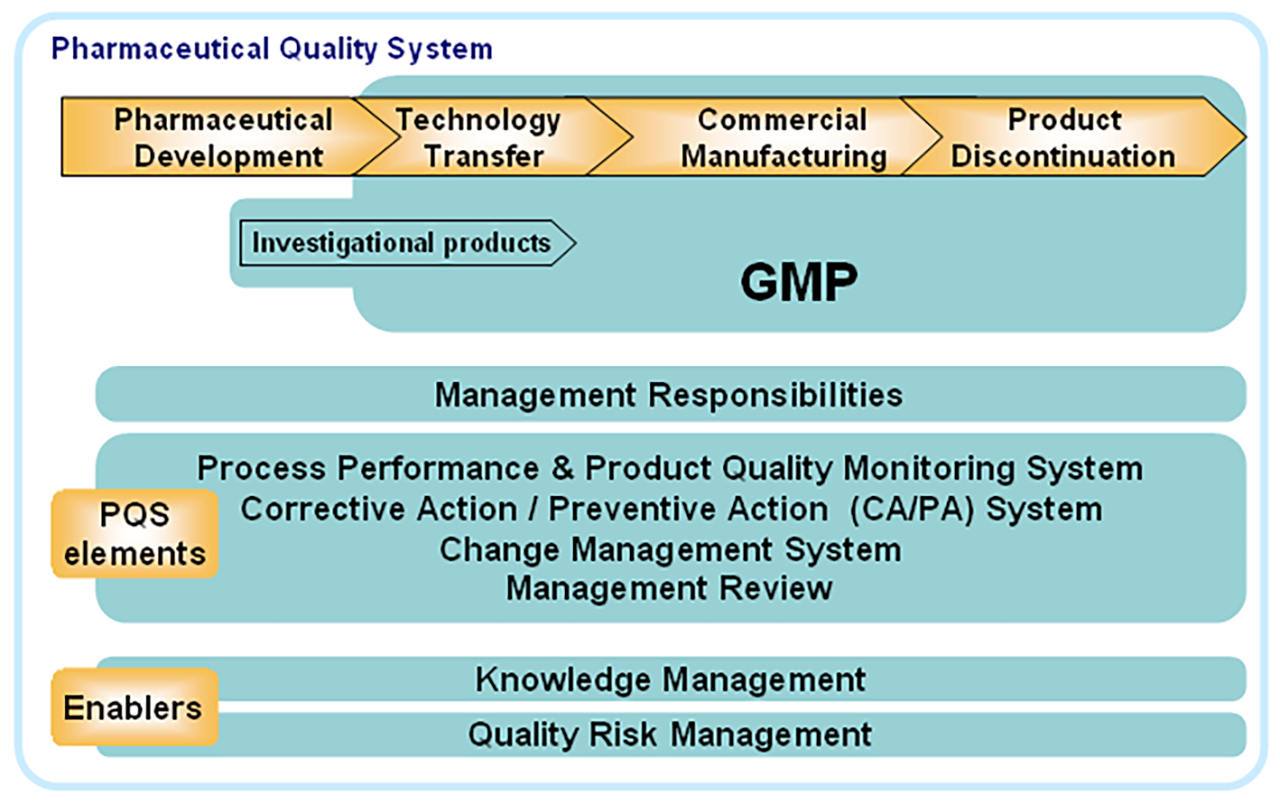
QRM is designed to sit within the PQS and influence and inform quality controls across the product lifecycle. It is not intended as a stand-alone guidance and was shortly supported by the publication in 2008 of ICH Q8: Pharmaceutical Development and ICH Q10: Pharmaceutical Quality System. The essence of the trilogy is that the critical-to-quality attributes of a product are identified early in the development lifecycle and that, with the correct application of the PQS, those attributes are be preserved and protected throughout the remainder of the product lifecycle. QRM and Knowledge Management (KM) are seen as enablers of this objective (Fig 1).
The specific objectives of ICH Q10 are to
- Achieve Product Realisation
To establish, implement, and maintain a system that allows the delivery of products with the quality attributes appropriate to meet the needs of patients, health care professionals, regulatory authorities (including compliance with approved regulatory filings) and other internal and external customers.
- Establish and Maintain a State of Control
To develop and use effective monitoring and control systems for process performance and product quality, thereby providing assurance of continued suitability and capability of processes. Quality risk management can be useful in identifying the monitoring and control systems.
- Facilitate Continual Improvement
To identify and implement appropriate product quality improvements, process improvements, variability reduction, innovations, and pharmaceutical quality system enhancements, thereby increasing the ability to fulfil a pharmaceutical manufacturer’s own quality needs consistently. Quality risk management can be useful for identifying and prioritising areas for continual improvement.
As a result of the trilogy, the future of pharmaceutical manufacturing was altered to one of process understanding, control, and improvement. Quality management and process management are intertwined. Management Review, and the role of management in general, has become critical to ensuring that the real-world outcomes of control are monitored and fed back to the source of control. Quality decisions re now made by the wider management group and are informed by QRM and KM. Specification limits have become the outer limit of ambition and real time process control limits provide greater assurance that downstream consequences of variability are minimised. Prevention of issues has become as important, if not more important, than reacting to them. Recurrence of issues became less acceptable. ICH Q9 and ICH Q10 were key influencers of these paradigm changes.
These ICH documents built on the lexicon of guidance’s published by the US FDA which were pivotal in establishing this approach – including guidance on Process Analytical Technology (PAT) in 2004, Current Good Manufacturing Practices (cGMP) for the 21st Century (2004), Quality by Design (QbD) (2009), and Emerging Technology (2017). Regulatory guidance had provided the required support for innovation and modernisation.
However, the modern PQS is much less site specific than even a decade ago, residing as it typically does within global organisations, that uses advancing technology across a diversified lifecycle. To remain relevant and for the PQS to remain effective its role in the complex environment of modern manufacturing operations is the focus of this research. Demonstrating the effectivity of the PQS is essential if an operation wants to avail of the regulatory reliefs offered in Annex 1 of ICH Q10 and ICH Q12.
Research Topics
- Establishing the Effectivity of QRM within the PQS
- The role of complexity its influence on the application of Quality Risk Management
- The role of QRM in informing decision making in the PQS
- Proactive application of the PQS in manufacturing environments
- The transition for Quality Control to Process Control
- RISK BASED DECISION MAKING
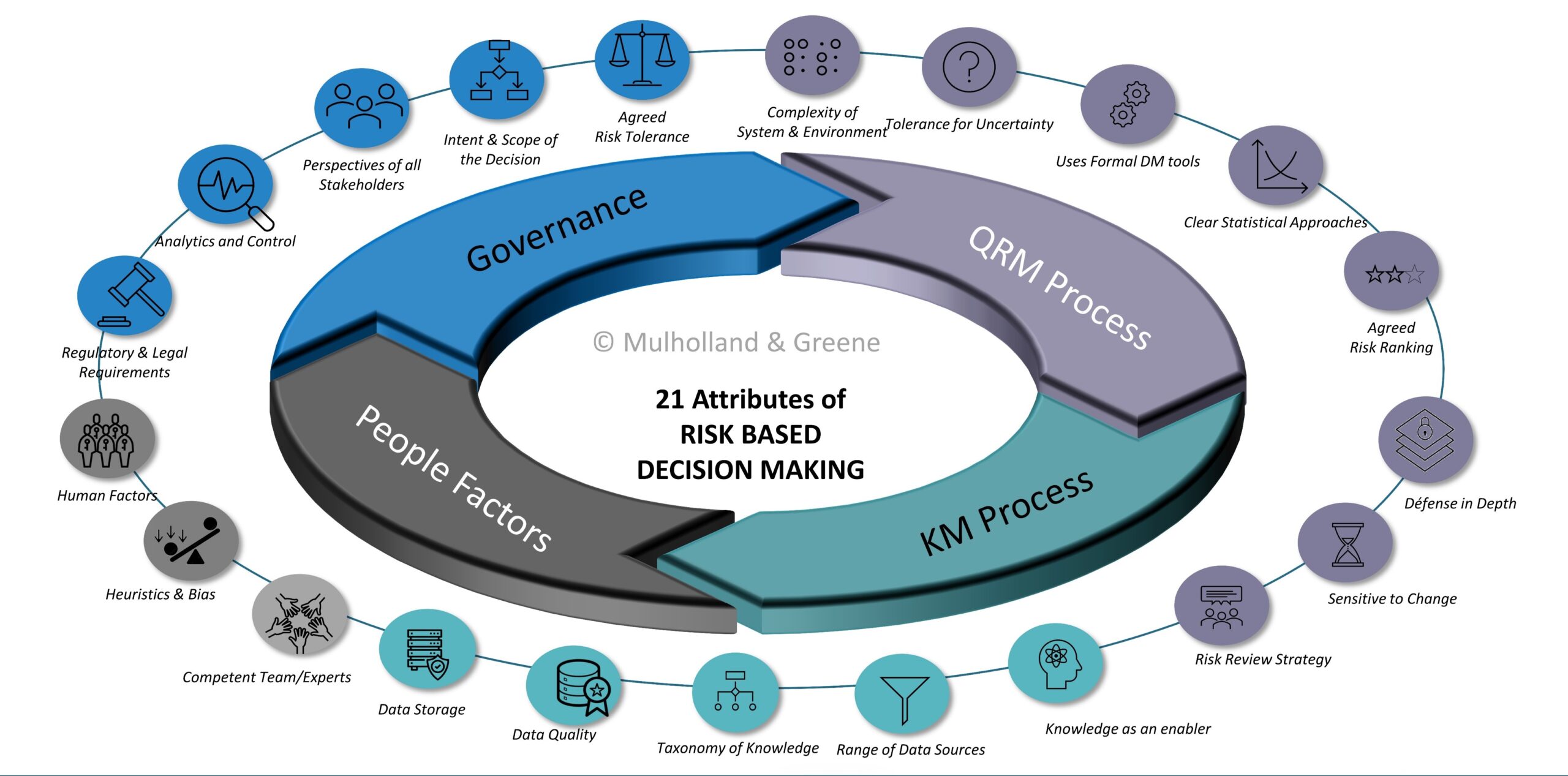
Risk Based Decision Making Publications
- Mulholland, Valerie; Greene, Anne (2023) ‘Improving RBDM Effectiveness: Insights from other Industries’ KENX Insight, Volume 3, Article 2 https://kenx.org/kenx_insights/improving-risk-based-decision-effectiveness-insights-from-other-industries-2/
- Mulholland, Valerie; Greene, Anne (2023) ‘Improving RBDM Effectiveness: A Case for Risk Decision Review Points’ KENX insight, Volume 3, Article 3 https://kenx.org/kenx_insights/improving-risk-based-decision-making-effectiveness-a-case-for-risk-decision-review-points-kdrps-in-the-quality-risk-management-process/
- Mulholland, Valerie: Greene, Anne (2023) ‘Improving RBDM Effectiveness: Determining the Level of Formality’ KENX Insight, Volume 3, Article 4 https://kenx.org/kenx_insights/improving-risk-based-decision-making-effectiveness-determining-the-level-of-formality/
- Mulholland, Valerie; Greene, Anne; Lipa, Martin J. (2023) ‘Improving RBDM Effectiveness: Addessing Uncertainty’ KENX Insight, Volume 3, Article 5 https://kenx.org/kenx_insights/improving-risk-based-decision-making-effectiveness-addressing-uncertainty/
- Mulholland, Valerie; Greene, Anne; and Lipa, Martin J. (2021) “Steps Beyond Risk Assessment in QRM: RBDM, The next horizon,” Level 3: Vol. 16: Iss. 1, Article 2.
- Mulholland, Valerie and Greene, Anne (2020) “Quality Risk Management: Seeking the Diamonds: Making the Case for Improved Formality in QRM Decision-making,” Level 3: Vol. 15: Iss. 2, Article 18. doi:https://doi.org/10.21427/yfw0-hy89
- Lipa, Martin; Mulholland, Valerie; and Greene (editor), Anne (2022) “Improving Risk-Based Decision Making (RBDM) Effectiveness Through a Framework which Integrates Knowledge and Risk,” Level 3: Vol. 16: Iss. 2, Article 2.
PQS Publications
- O’Donnell K., Tobin D., Butler S., Haddad G., Kelleher D., (2020) Understanding the Concept of Formality in Quality Risk Management. IVT Netwrk.com (online)
https://www.ivtnetwork.com/article/understanding-concept-formality-quality-risk-management - Mulholland V., Warreth, S. (2020) Risk Management Ties up with a Bow Tie. Knex Insight (online)
- Greene, A., O’Donnell, K., Murphy, A. and Harris, E. (eds). (2019). An audience with Pharmaceutical Regulators, Academia and Industry: The Role of Quality Risk Management (QRM) and Knowledge Management (KM) in medicinal product realisation for patient in the 21st century. Dublin: TU Dublin Academic Press.
- Greene, A., O’Donnell, K., Calnan, N. (eds). (2018). An audience with international regulators in the manufacture of medicines: quality risk management (QRM) and knowledge management (KM). Dublin: DIT Academic Press. isbn:978 1 90045467 X doi:10.21427/D7SZ5W
- Waldron K, (2017) Managing Risk to the Patient: Recoding Quality Risk Management for the Pharmaceutical and Biopharmaceutical Industries. Doctoral Thesis DIT
- Vesper J., O’Donnell K. (2016) Current Challenges in Implementing Quality Risk Management Pharmaceutical Engineering 2016:73-9
- Waldron K., Greene A., Calnan N., (2015) Quality Risk Management, The State of the Industry – Part 1. Has the Industry realised the full value of ICH Q9? Journal of Validation technology Vol 20, No 4
- Calnan N., O’Donnell K., Greene A., (2013) Enabling ICH Q10 Implementation: Part 1 Striving for Excellence by Embracing ICH Q8 and ICH Q9, PDA J. Pharm Sci. Techno. 2013, Vol 67, no 6, p1-20
- O’Donnell, Kevin. (2007). The development of a Quality Risk Management solution designed to facilitate compliance with the risk-based qualification, validation and change control GMP requirement of the EU. Technological University Dublin. doi:10.21427/D7C311
- Waldron, (2018) “Quality Risk Management 101: ICH Q9 In Context,” Pharmaceutical Online, 28 March 2018. [Online].
Resources
- ICH Q9, Quality Risk Management, https://ich.org/page/quality-guidelines
- ICH Q8(R1), Pharmaceutical Development, https://ich.org/page/quality-guidelines
- ICH Q10, Pharmaceutical Quality System, https://ich.org/page/quality-guidelines
- ICH Q11, Development and Manufacture of Drug Substances, https://ich.org/page/quality-guidelines
- ICH Q12, Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management, https://ich.org/page/quality-guidelines
- ISO Standard 31000:2009 Risk Management – Principles and Guidelines, https://www.nsai.ie/standards/sectors/risk-management/
- ICH Q9 Briefing Pack, https://ich.org/page/quality-guidelines
- WHO Guideline on Quality Risk Management, Document No. QAS/10.376, August 2010, https://www.who.int/medicines/services/expertcommittees/pharmprep/Qualit.
- Food and Drug Administration, “Guidance for Industry: PAT A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance,” FDA, 2004.
- Food and Drug Administration, “Pharmaceutical cGMP for the 21st Century, a Risk Based Approach,” FDA, 2004.
- Food and Drug Administration, “Advancement of Emerging Technology Applications for Pharmaceutical Innovation and Modernisation, Guidance for Industry,” FDA, 2017.