Knowledge Management

Risk-Knowledge Infinity Cycle
Link to related publication:
Knowledge as the Currency of Managing Risk: A Novel Framework to Unite Quality Risk Management and Knowledge Management
Introduction
Knowledge Management (KM) is critical to the biopharmaceutical industry and the patients it serves as the industry is one based on knowledge. Knowledge about products, processes, platforms and components. Knowledge about risks and how to mitigate them. Knowledge about what happened in the past, failed experiments and why decisions were made. Knowledge in the form of ‘know how’, experience and expertise. And much more.
The recognition of the importance of Knowledge Management has grown significantly in recent years, sparked by the promise of leveraging ‘prior knowledge’ in the regulatory guidance document Pharmaceutical Development, ICH Q8 in 2005. This prominence grew larger when Knowledge Management was positioned as an enabler to an effective Pharmaceutical Quality System in ICH Q10 in 2008.
Knowledge Management is defined by ICH Q10 as:
“a systematic approach to acquiring, analysing, storing, and disseminating information related to products, manufacturing processes and components.”
This definition is important yet arguably somewhat narrow in scope and infers a bias toward only documented knowledge (i.e. ‘explicit’ knowledge). Over time, additional definitions of KM have emerged which provide a more holistic lens on knowledge management, inclusive of experience and know how (i.e. ‘tacit’ knowledge), such as the definition by APQC (apqc.org),
“applying systematic approaches to help information and knowledge flow to the right people, at the right time, in the right format and at the right cost, so they can act more efficiently and effectively to create value.”
And while ISO did not issue a standard on Knowledge Management until 2018, it also provides a helpful definitions of Knowledge and Knowledge Management. Knowledge is defined as
“Human or organizational asset enabling effective decisions and action in context. Knowledge can be individual, collective or organizational. There are diverse views on the scope covered within knowledge, based on context and purpose. The definition above is general as to the various perspectives. Examples of knowledge include insights and know-how. Knowledge is acquired through learning or experience.”
And Knowledge Management is defined as
“Management with regard to knowledge. It uses a systemic and holistic approach to improve results and learning. It includes optimizing the identification, creation, analysis, representation, distribution and application of knowledge to create organizational value.”
However, tangible progress in knowledge management has been slow and elusive for the industry. As such, the PRST has engaged in research directed at creating a better understanding of what Knowledge Management is, why it is important and how organizations can advance in ‘how they manage their knowledge as an asset.’ The PRST has also advanced the dialogue between industry, regulatory agencies and academia through a number of symposia and conference presentations. Following is a high-level guide to research conducted by the PRST and related publications and conference proceedings, followed by helpful references on these studies.
PRST research commenced in 2011 led by Nuala Calnan with a focus on enhancing pharmaceutical product quality. Calnan’s 2015 thesis, Protecting the Patient: Enhancing the Quality of Pharmaceutical Products explored the unacceptable risks that patients are exposed to due to challenges which exist in the complex pharmaceutical product lifecycle.
Calnan’s exploration in knowledge management as an orphan enabler to the pharmaceutical quality system established the foundations for PRST in knowledge management. Calnan reviewed the concepts of tacit and explicit knowledge, the concept of knowledge flow and the link between the importance of capturing and applying knowledge to an effective pharmaceutical quality system. Calnan also proposed that the elements of the pharmaceutical quality system (product performance and product quality monitoring system, CAPA, change management and management review) should operate through well-integrated and balanced enablers of knowledge management and quality risk management.
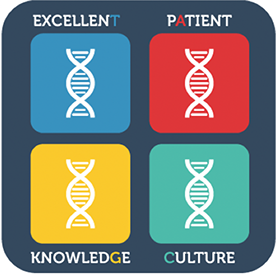
Calnan explored the topics of being excellent and cultural excellence, in addition to the aforementioned knowledge excellence focus. A key output from the research was a construct of the Building Blocks of Cultural DNA of Quality (see figure), in which these elements of knowledge excellence, cultural excellence, patient focus and being excellent converge to create a culture of quality.
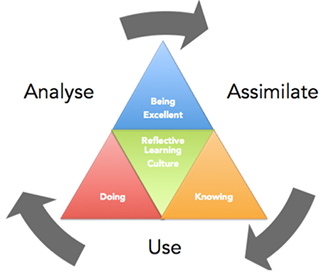
The primary research output of Calnan’s work was the Excellence Framework (see figure), a combination of the cultural excellence of a learning organization with excellence in knowledge creation and utilization, in order to deliver operational excellence based on a relentless restlessness for improvement. The model is dynamic and encourages one to analyse the current situation for improvement opportunities, to assimilate the findings to create new knowledge and then use to take action, decisions and deliver solutions. Reflection is promoted to confirm the effectiveness of the action taken and share lessons learned.
KM Publications
- Enabling ICH Q10 Implementation—Part 1. Striving for Excellence by Embracing ICH Q8 and ICH Q9 (Article, Nov 2013)
- Knowledge Management, ISPE Pharmaceutical Engineering e-Supplement (Journal, May 2014)
- An Investment in Knowledge Pays the Best Interest—Are You Leveraging Your Investment in Your CTDs by Using the Knowledge? (Article, Sep 2015)
- A Lifecycle Approach to Knowledge Excellence in the Biopharmaceutical Industry (Book, Jul 2017)
- A Blueprint for Knowledge Management in the Biopharmaceutical Sector (Doctoral Thesis, Oct 2018)
- An Audience with International Regulators in the Manufacture of Medicines: Quality Risk Management (QRM) and Knowledge Management (KM) (Monograph, Oct 2018)
- An Audience with Pharmaceutical Regulators, Academia and Industry 2019: the Role of Quality Risk Management (QRM) and Knowledge Management (KM) in Medicinal Product Realisation for Patients in the 21st. Century (Monograph, Apr 2019)
- Effective Knowledge Transfer During Biopharmaceutical Technology Transfer – How Well Do We Do It? (Article, Aug 2019) & Winner, Journal of Validation Technology Author of the Year Award
- Advancing Knowledge Management (KM) as an ICH Q10 Enabler in the Biopharmaceutical Industry (Monograph, May 2020)
- Knowledge Management Implementation: A Survey of the Biopharmaceutical Industry (Article, May 2020)
- Knowledge Management as a Pharmaceutical Quality System Enabler: How Enhanced Knowledge Transfer can help close the Q10 to Q12 Gap (Article, Jul 2020)
- Managing Knowledge and Risk – A Literature Review on the Interdependency of QRM and KM as ICH Q10 Enablers (Article, Aug 2020)
- Knowledge as an Asset in Pharmaceuticals (Podcast, Sep 2020)
- Knowledge as the Currency of Managing Risk: A Novel Framework to Unite Quality Risk Management and Knowledge Management (Article, Oct 2020)
Guidance Documents
- ICH Q10, Pharmaceutial Quality System
- Quality Implementation Working Group on Q8, Q9 and Q10 Questions & Answers
- ISO 30401:2018 Knowledge management systems — Requirements
- ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry (in development)
KM Conference Presentations
- TBC
Other Helpful Links
Building blocks of the cultural DNA of quality
The Excellence Framework
KM research continued by Paige Kane, culminating with the 2018 thesis on A Blueprint for Knowledge Management in the Biopharmaceutical Sector. Kane’s focus was on exploring the level of adoption and capability of knowledge management in the biopharmaceutical sector. In finding a general lack of maturity in the sector, Kane’s research produced a collection of assets for the sector to leverage to unlock their knowledge.
The primary output of Kane’s research was a new framework entitled the Pharma KM Blueprint (see figure). This framework provided a construct of principles, models and tools summarized as follows:
- Managing Knowledge as an Asset – addresses the need to value and maintain knowledge assets in the same way as physical assets within an organization.
- The Pharmaceutical Product Knowledge Lifecycle Model (PPKL) (see figure) addresses the challenge of enabling knowledge flow in order to increase visibility, to access and use the product and process knowledge assets across the product lifecycle.
- The House of Knowledge Excellence Framework (HOKE), which demonstrates a framework developed to implement a systematic knowledge management programme linked to strategic objectives of an organisation, incorporating knowledge management practices, pillars (people, process, technology, governance), and enablers to support the effective management and flow of knowledge assets.
- A Knowledge Management Effectiveness Evaluation, which provides a practical knowledge management diagnostic tool that may be used to identify and evaluate areas of opportunity and to track progress on closing knowledge gaps.
The features of the PPKL model include:
- The vision for end-to-end (E2E) product and process knowledge asset visibility, transparency and availability in order to enable knowledge flow of critical knowledge to those that need it throughout the product lifecycle.
- The addition of a new lifecycle phase of New Product Introduction (NPI) to replace the Technology Transfer lifecycle phase.
- That Technology Transfer is an activity that may occur multiple times across the product lifecycle.
- The addition of a new E2E process to capture the Technical Product Support and Continual Improvement activities that occur across the product lifecycle.
Additional information on the House of Knowledge Excellence can be found in the referenced publication below, A Lifecycle Approach to Knowledge Excellence in the Biopharmaceutical Industry.
PRST research continued with an additional study initiated in 2018 by Martin Lipa. Lipa’s study explored how knowledge is managed across the product lifecycle, with a specific focus on technology transfer and associated transfer of tacit knowledge. Lipa’s efforts also included an assessment of the under-appreciated relationship between knowledge management and quality risk management (QRM), in an attempt to improve quality risk management outcomes and in turn enhance patient protection.
Lipa’s study which is currently ongoing has generated several key insights and outputs. Among them, after a confirmation that knowledge transfer during technology transfer requires improvement, is a proposed framework for technology transfer knowledge transfer enhancement, the KTE Framework (see Figure). This framework features a closed-loop process akin to a Plan-Do-Check-Act cycle to enhance knowledge transfer, as a means to preserve, transfer and knowledge and achieve ICH Q10 objectives of product realization, a state of control and a basis for continual improvement. The beginnings of an associated KTE Toolkit is also being developed, presenting a collection of KM practices and tools to support execution of the KTE Framework.
Through examination of the QRM-KM relationship, Lipa’s research has explored that knowledge is both an input and an output to an effective QRM process, and that effective KM can help provide the best possible knowledge to QRM and enable the best possible risk reduction. The key output of this examination was a framework entitled the Risk-Knowledge Infinity Cycle. This framework illustrates that increasing knowledge leads to decreasing risk and that this process is continuous and perpetual. Lipa subsequently applied this framework to ICH Q10 to depict the relationship between QRM and KM (see Figure).
KM Publications
- Enabling ICH Q10 Implementation—Part 1. Striving for Excellence by Embracing ICH Q8 and ICH Q9 (Article, Nov 2013)
- Knowledge Management, ISPE Pharmaceutical Engineering e-Supplement (Journal, May 2014)
- An Investment in Knowledge Pays the Best Interest—Are You Leveraging Your Investment in Your CTDs by Using the Knowledge? (Article, Sep 2015)
- A Lifecycle Approach to Knowledge Excellence in the Biopharmaceutical Industry (Book, Jul 2017)
- A Blueprint for Knowledge Management in the Biopharmaceutical Sector (Doctoral Thesis, Oct 2018)
- An Audience with International Regulators in the Manufacture of Medicines: Quality Risk Management (QRM) and Knowledge Management (KM) (Monograph, Oct 2018)
- An Audience with Pharmaceutical Regulators, Academia and Industry 2019: the Role of Quality Risk Management (QRM) and Knowledge Management (KM) in Medicinal Product Realisation for Patients in the 21st. Century (Monograph, Apr 2019)
- Effective Knowledge Transfer During Biopharmaceutical Technology Transfer – How Well Do We Do It? (Article, Aug 2019) & Winner, Journal of Validation Technology Author of the Year Award
- Advancing Knowledge Management (KM) as an ICH Q10 Enabler in the Biopharmaceutical Industry (Monograph, May 2020)
- Knowledge Management Implementation: A Survey of the Biopharmaceutical Industry (Article, May 2020)
- Knowledge Management as a Pharmaceutical Quality System Enabler: How Enhanced Knowledge Transfer can help close the Q10 to Q12 Gap (Article, Jul 2020)
- Managing Knowledge and Risk – A Literature Review on the Interdependency of QRM and KM as ICH Q10 Enablers (Article, Aug 2020)
- Knowledge as an Asset in Pharmaceuticals (Podcast, Sep 2020)
- Knowledge as the Currency of Managing Risk: A Novel Framework to Unite Quality Risk Management and Knowledge Management (Article, Oct 2020)
Guidance Documents
- ICH Q10, Pharmaceutial Quality System
- Quality Implementation Working Group on Q8, Q9 and Q10 Questions & Answers
- ISO 30401:2018 Knowledge management systems — Requirements
- ISPE Good Practice Guide: Knowledge Management in the Pharmaceutical Industry (in development)
KM Conference Presentations
- TBC